26+ Why Are Halogens So Reactive
Halogens are so reactive because they have a valence. Web Why Are Halogens So Reactive.
The Halogens Halide Ions And Their Reactions A Level Chemistry Study Mind
As a general rule fluorine is the most reactive halogen and.

. This is the opposite trend to that seen in the alkali metals in Group 1 of. Web Halogens actually are not all gases At is a solid. A more reactive halogen will displace a less reactive halide from an aqueous solution known as a.
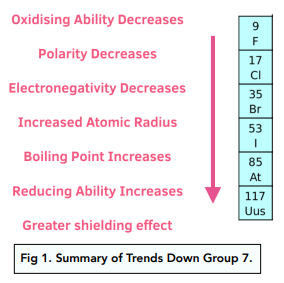
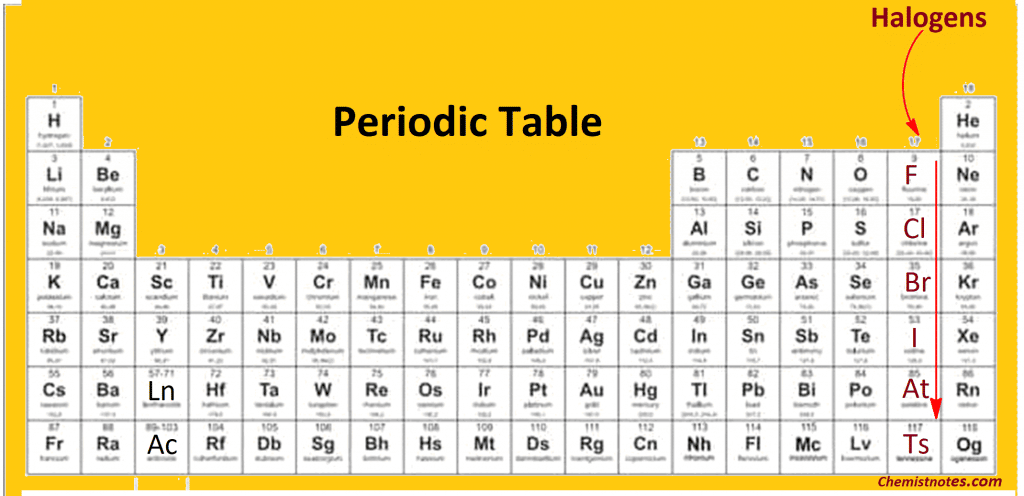
Web The halogens. Web Halogens are among the most reactive of all elements however reactivity declines from the top to the bottom of the halogen group. Including fluorine F chlorine Cl bromine Br iodine I thorium At Tennessine.
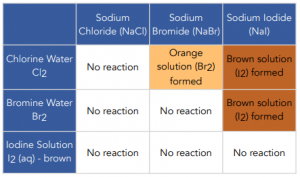
Web the most reactive halogen displaces all of the other halogens from solutions of their salts. Web Halogens are extremely reactive and they form bonds with other elements easily. Halogens are highly reactive as.
Web Theyre only one electron away from achieving from satisfying the octo rule. Therefore they are highly reactive and can gain an. Therefore they will very aggressively seek out that one extra electron so that they can form an octet.
Web A halogen element refers to an element of Group A of the periodic system. To the question are halogens reactive the answer is yes and the reason lies in their electron configuration. The iodide ions have lost electrons so they have been oxidised.
Since all halogens have seven. Web However halogens readily combine with most elements and are never seen uncombined in nature. So it has been reduced.
Web Why Halogens are So Reactive. They are so reactive because they have 7 valence electrons so they are very close to fulfilling the octet rule. Web Answer and Explanation.
All the halogens except iodine are found in nature as salts of the halide ions X so the. Web Preparation and General Properties of the Group 17 Elements. This reactivity is attributed to high.
They are so reactive because they all have 7 valence. Atoms of all the. Noble gases are all gases.
Halogen has the general electronic configuration ns 2. Web understand why halogens are so reactive. - Halogens are extremely reactive and can be toxic or lethal in appropriate amounts to biological species.
Web Due to increased strength of Van der Waals forces down the group the boiling points of halogens increase. Web The oxidising ability of halogens decreases as you move down the group. Fluorine chlorine bromine and iodine are the most reactive non-metals on the Periodic Table.
Web The non-metal elements in Group 7 known as the halogens get less reactive as you go down the group. Chemically halogens are extremely reactive whereas noble gases dont react at all it. Be able to balance.
Halogens General Characteristics Physical Properties Chemistry Notes
Why Are Halogens Very Reactive Quora

Why Are Group 17 Halogens Highly Reactive Quora
2 22 Halogens Chemistry Libretexts
The Halogens Reactions With Halogens A Level Chemistry Study Mind
Halogens Chemistry Encyclopedia Uses Elements Gas Number Name Symbol Salt Atom
Phycology Free Full Text Halogens In Seaweeds Biological And Environmental Significance

2 22 Halogens Chemistry Libretexts
How Does Acetylene React With Halogen Quora

Reactions Of Halogens Explanation Examples Facts
2 22 Halogens Chemistry Libretexts
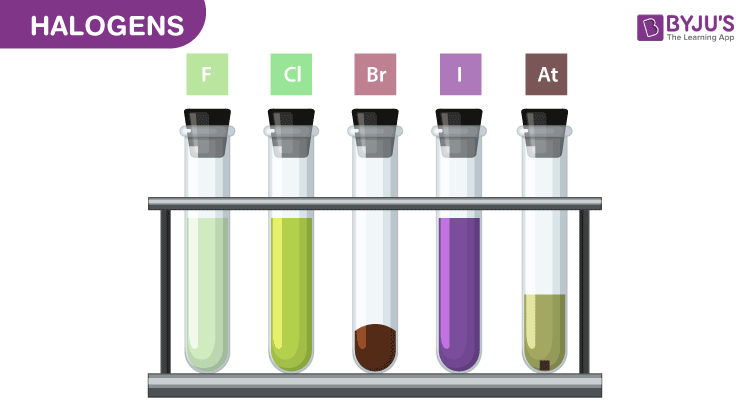
Halogens Trends In Chemical And Physical Properties Halogens Reactive

Why Are Halogens Very Reactive Quora
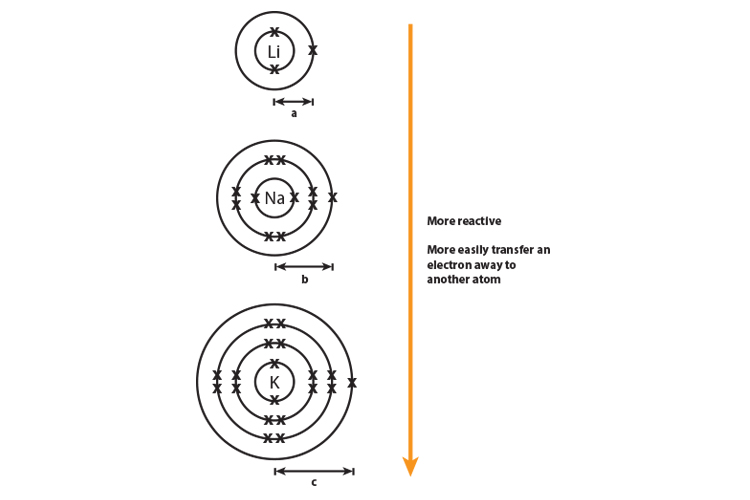
As You Move Down Group 1 And 7 Elements Get More Reactive

Group 17 The Halogens

Halogens Definition Uses Properties Elements I Studysmarter

Periodic Table Extract Showing The Non Metallic Elements The Groups Download Scientific Diagram